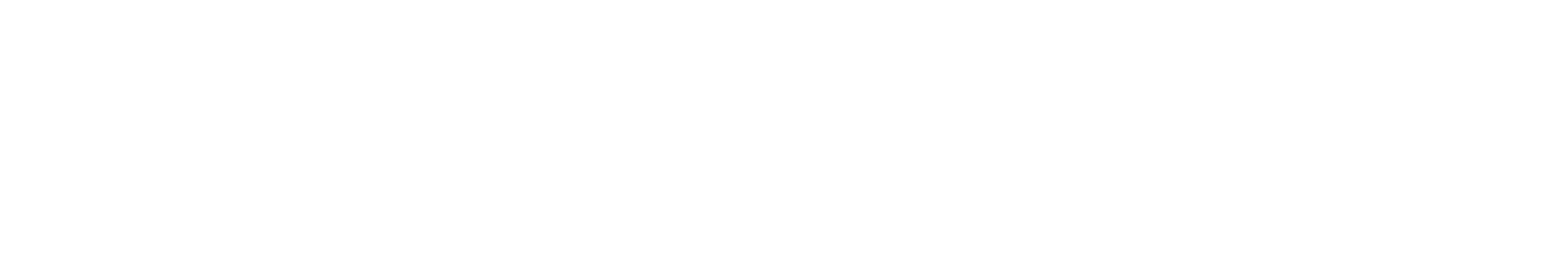Integra LifeSciences Sales
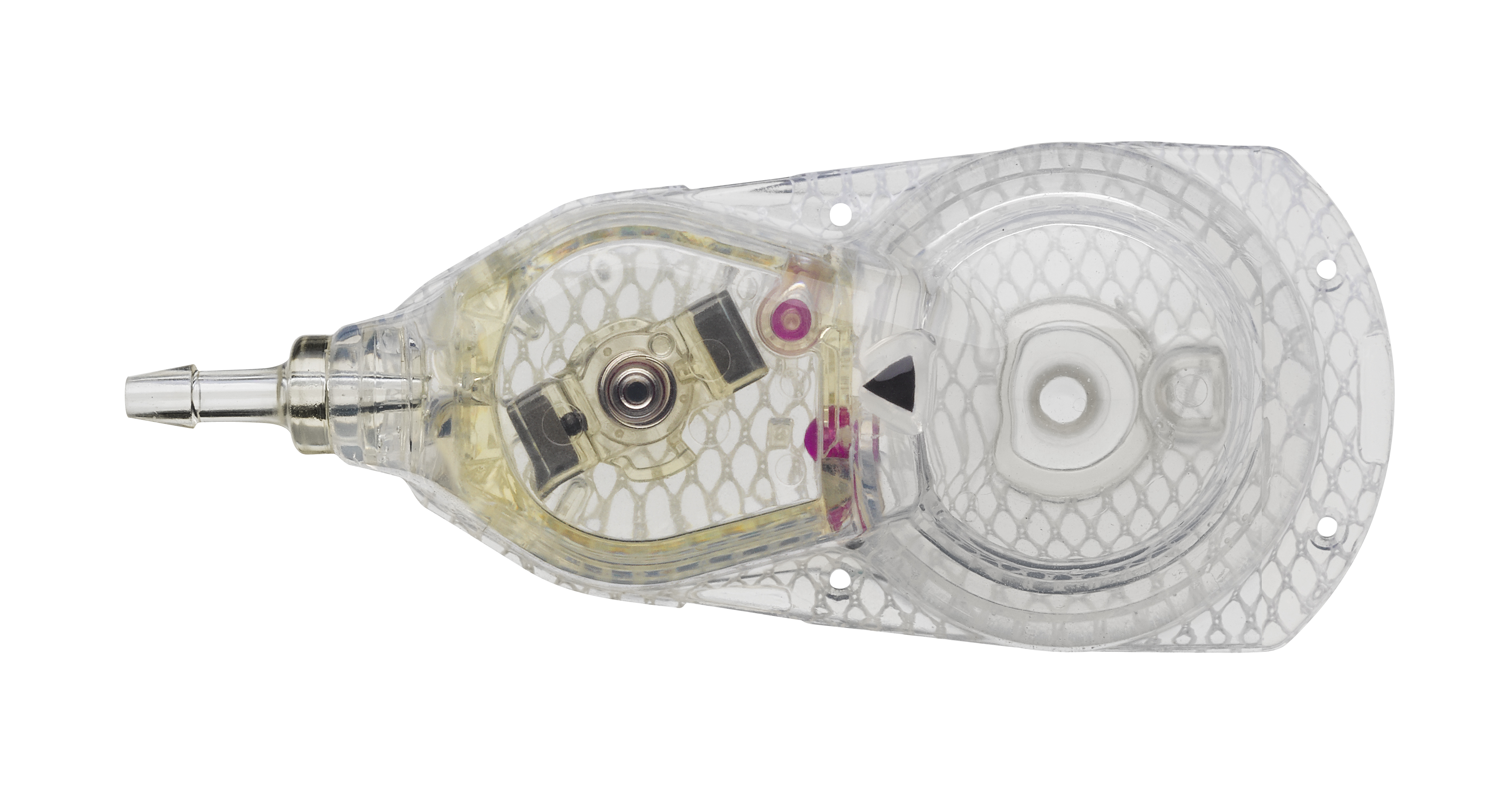
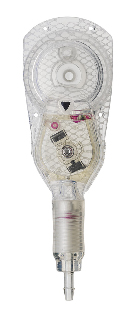
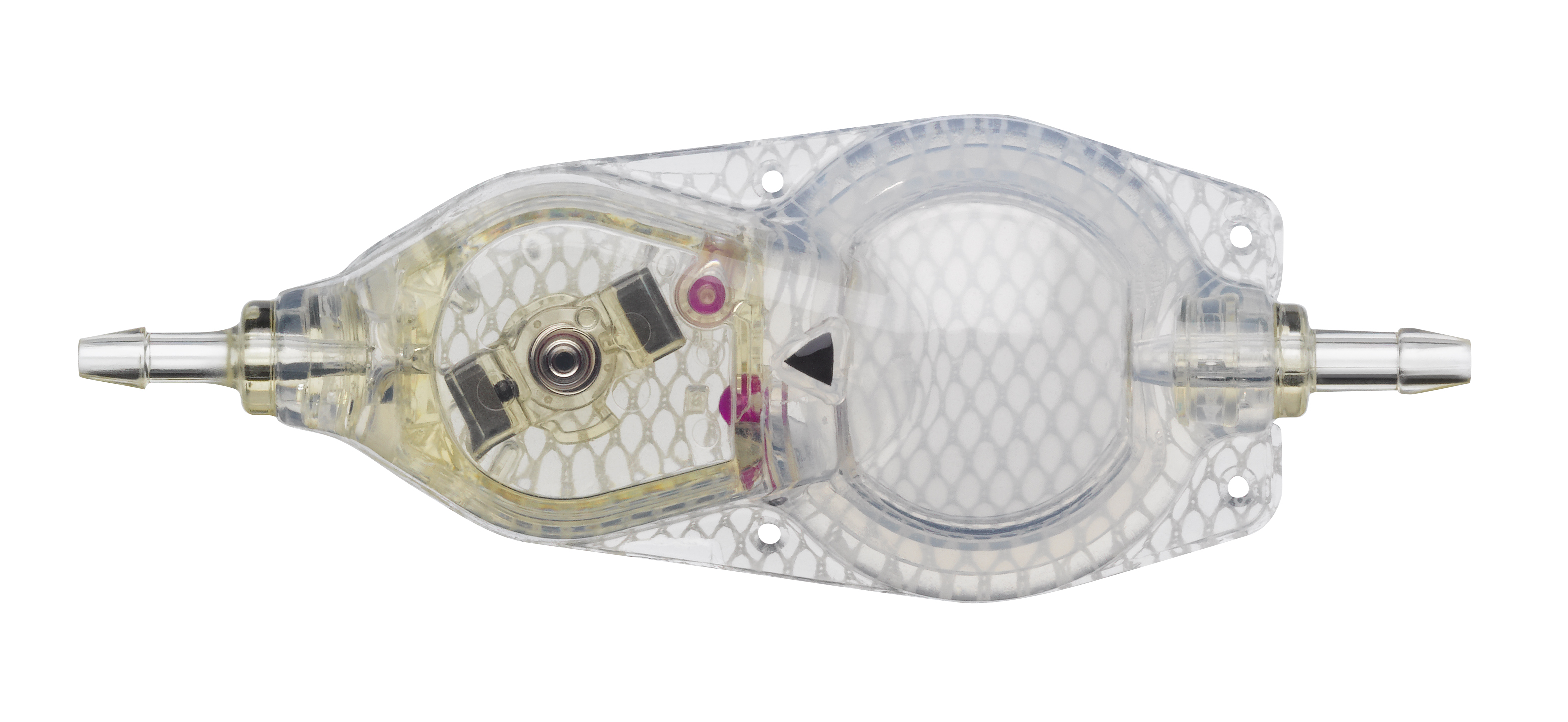
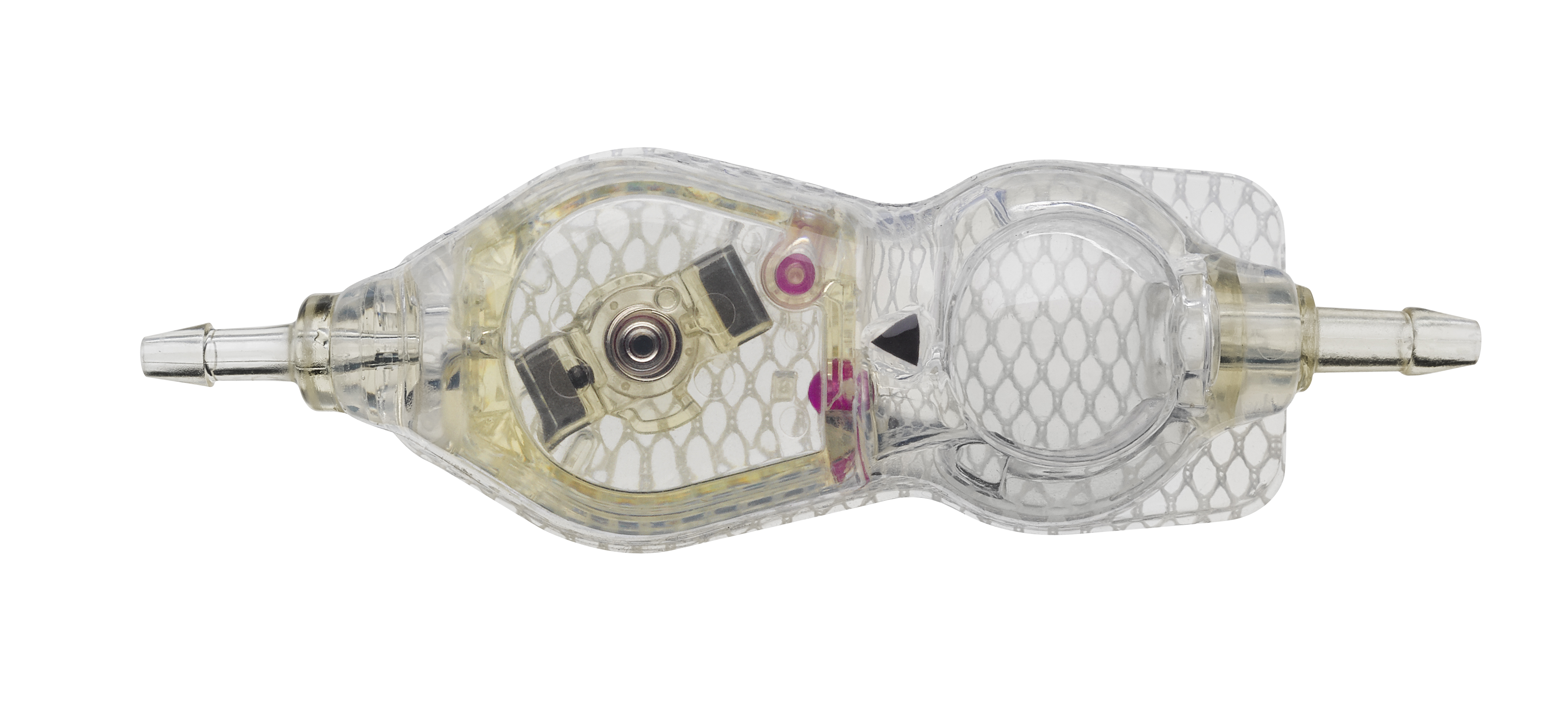
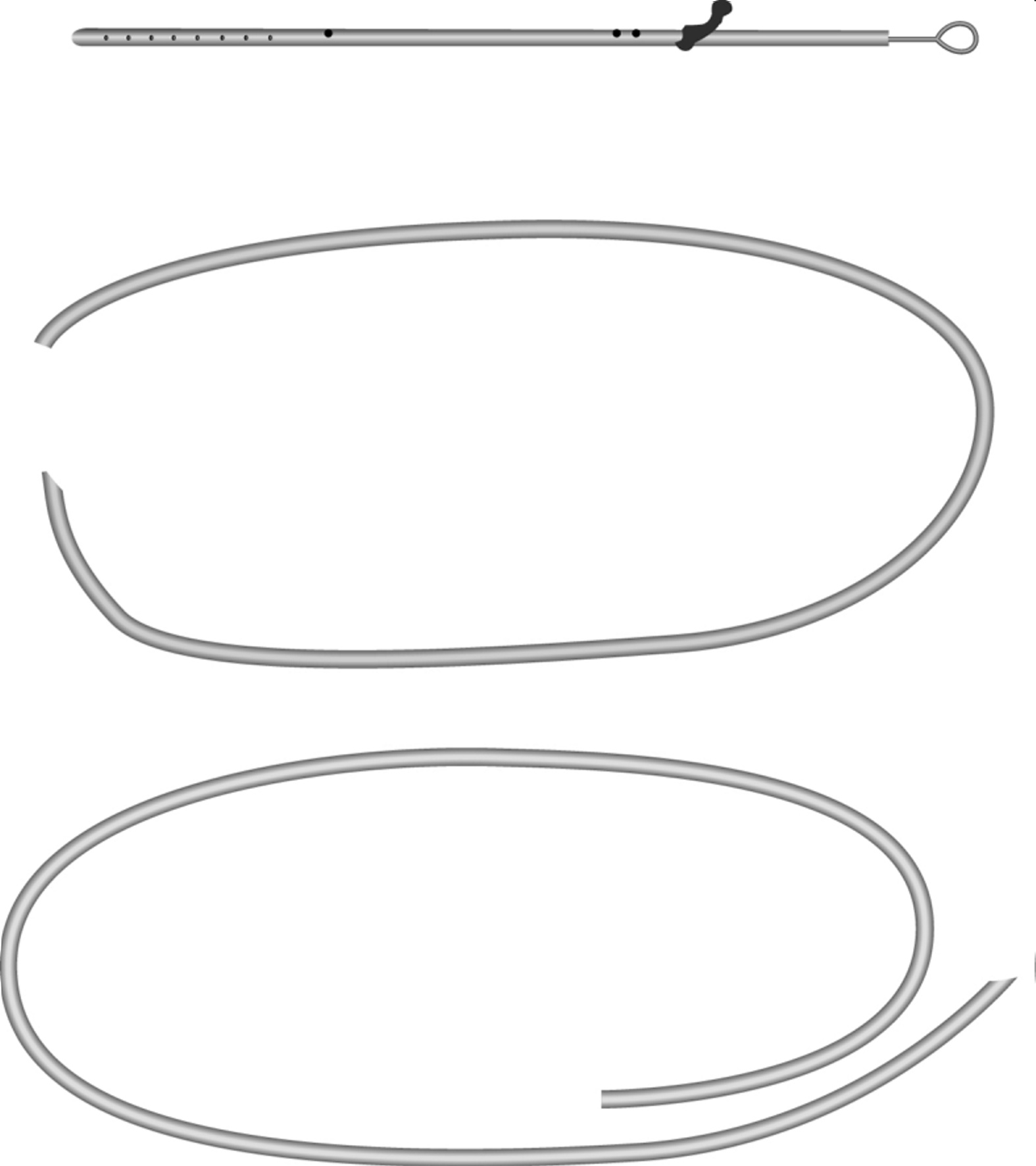
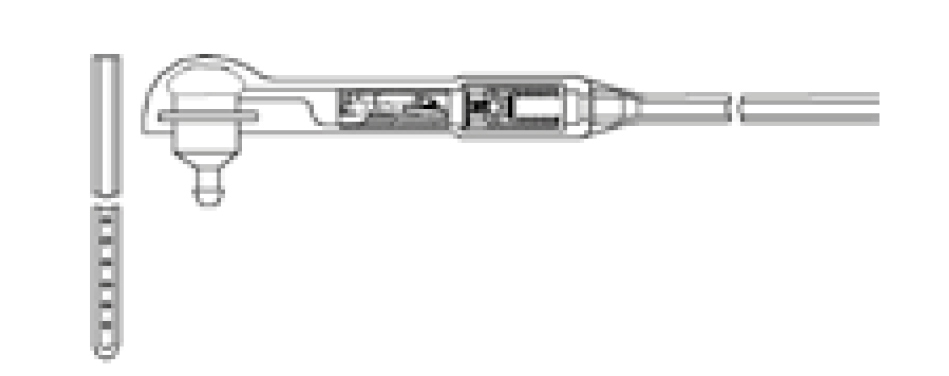
The Codman® CERTAS™ Plus programmable valve offers the ability to optimize the opening pressure of a shunt system before and after implantation. A shunted patient’s condition will often change over the course of their treatment making pressure changes necessary. The Codman® CERTAS™ Plus programmable valve allows a surgeon to non-invasively change the opening pressure (from 25 to 215 mm H2 O) to one of eight performance settings, which range from 1 (low pressure) to 7 (high pressure), plus performance setting 8, which is a “Virtual Off.” The setting of the Codman® CERTAS™ Plus programmable valve is changed through the use of an externally applied magnetic field. Applying a specific magnetic field to the adjustable valve mechanism will permit the cam to turn slightly, increasing or decreasing the tension on the spring, and changing the setting of the valve. To avoid unintended pressure setting changes, the valve is designed to withstand external magnetic influences, including MRI up to 3 Tesla. The Codman® CERTAS™ Plus programmable valve is available in 3 different designs (regular, small and right angle) and with integrated SiphonGuard® antisiphon d