IV Fixation
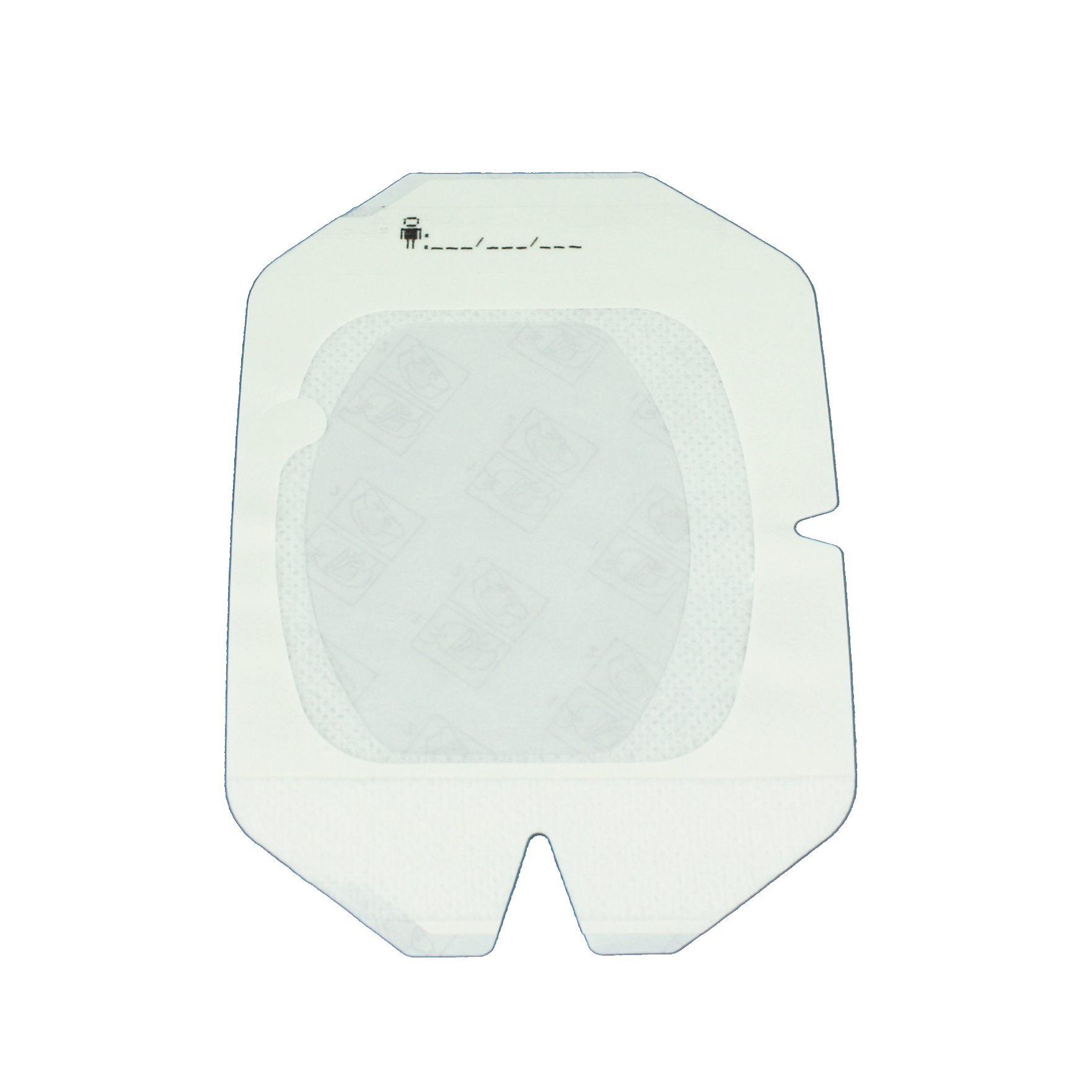
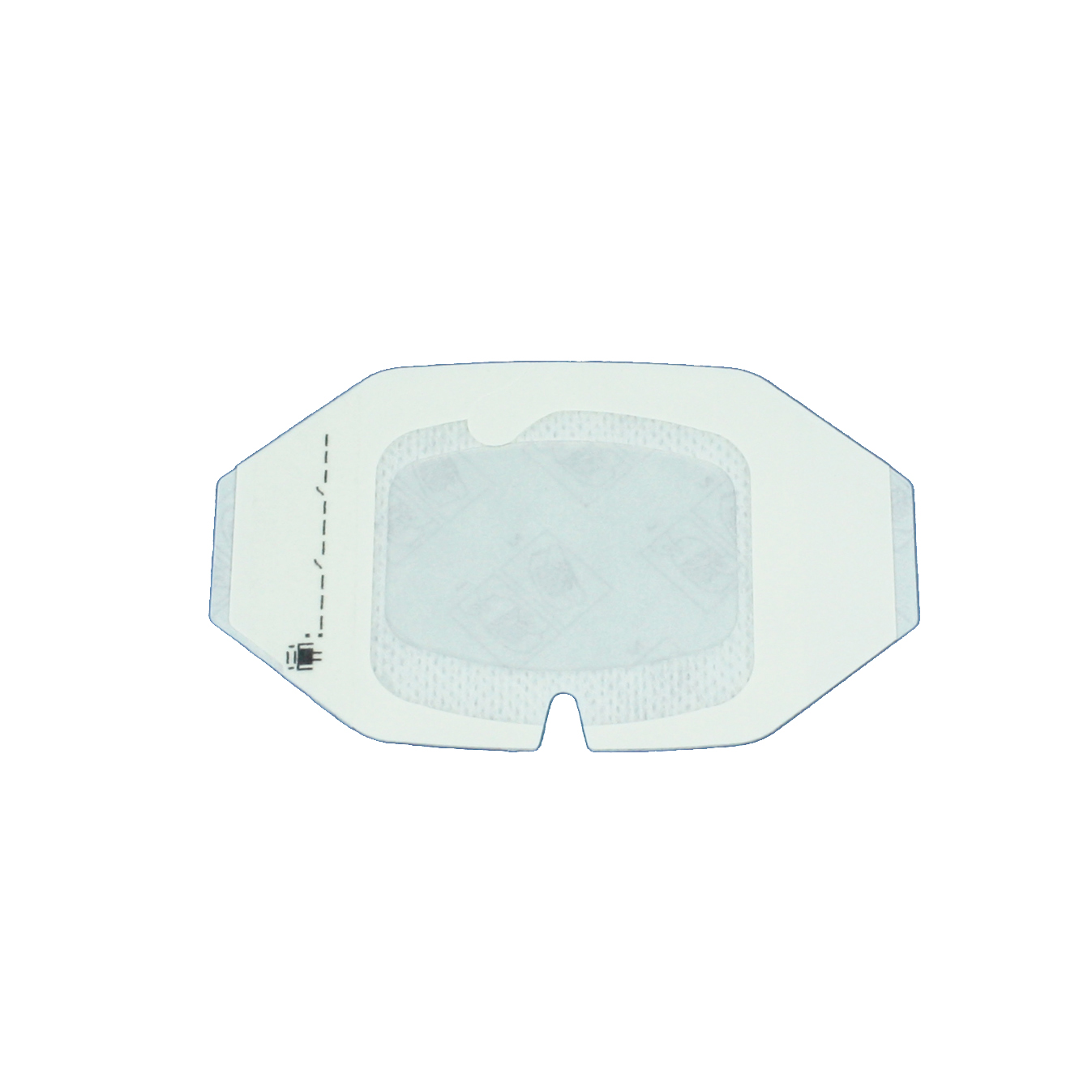
At Mediplast we are now launching our new IV fixation dressings. The dressings consist of semi-permeable film which is air-permeable whilst preventing bacteria and viruses from reaching the needle entry point. The dressings have a transparent surface which facilitates inspection of the needle entry point, as well as fixation tape and a frame system designed to aid application.
IV-dressing
Mediplast can offer IV fixation dressings. The transparent film makes inspection of the point of needle entry possible and the frame system enables easy handling and application. The dressings have hypoallergenic adhesive* in order to minimize the risk for skin irritation. The transparent film has a bacterial barrier**, it is water repellant** and semi permeable** which allows the skin to breathe.
*According to: EN ISO 10993 Biological evaluation of medical devices
**The products are tested according to the following standards:
EN 13726-2 MVTR
EN 13726-3 Waterproofness
EN 13726-4 Conformability
EN 13726-5 Microbial barrier
EN 13726-6 Odour control
Medical Device Classification: Is
| Item Nr | Article Name | Specifications | Packing size / Unit |
| 60702102 | IV-fixation dressing transparent | 7x8,5cm with frame | 100 / PCE |
| 60702103 | IV-fixation dressing transparent | 10x12cm with frame | 100 / PCE |
| 60702104 | IV-fixation dressing transparent | 8,5x11,5cm with frame | 50 / PCE |
| 60702105 | IV-fixation dressing transparent | 10x15,5cm with frame | 50 / PCE |
| 60702106 | IV-fixation dressing transparent | 6x7cm with frame | 100 / PCE |
| 60702107 | IV-fixation dressing transparent | 8,5x10,5cm with frame | 100 / PCE |
| 60702108 | IV-fixation dressing transparent | 7x9cm with frame | 50 / PCE |
| 60702101 | IV-fixation dressing transparent | 5x5,7cm with frame child | 100 / PCE |
We adhere to applicable European regulations and standards governing medical devices, ensuring full compliance with regulatory requirements.
| Document Name | File Size | File Type |